S D And P Orbitals
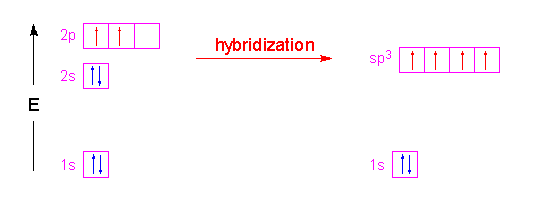
Formation of a hydrogen molecule from two hydrogen atoms.
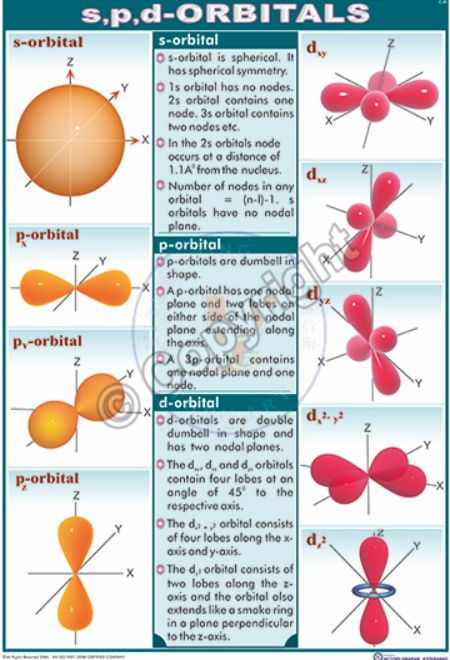
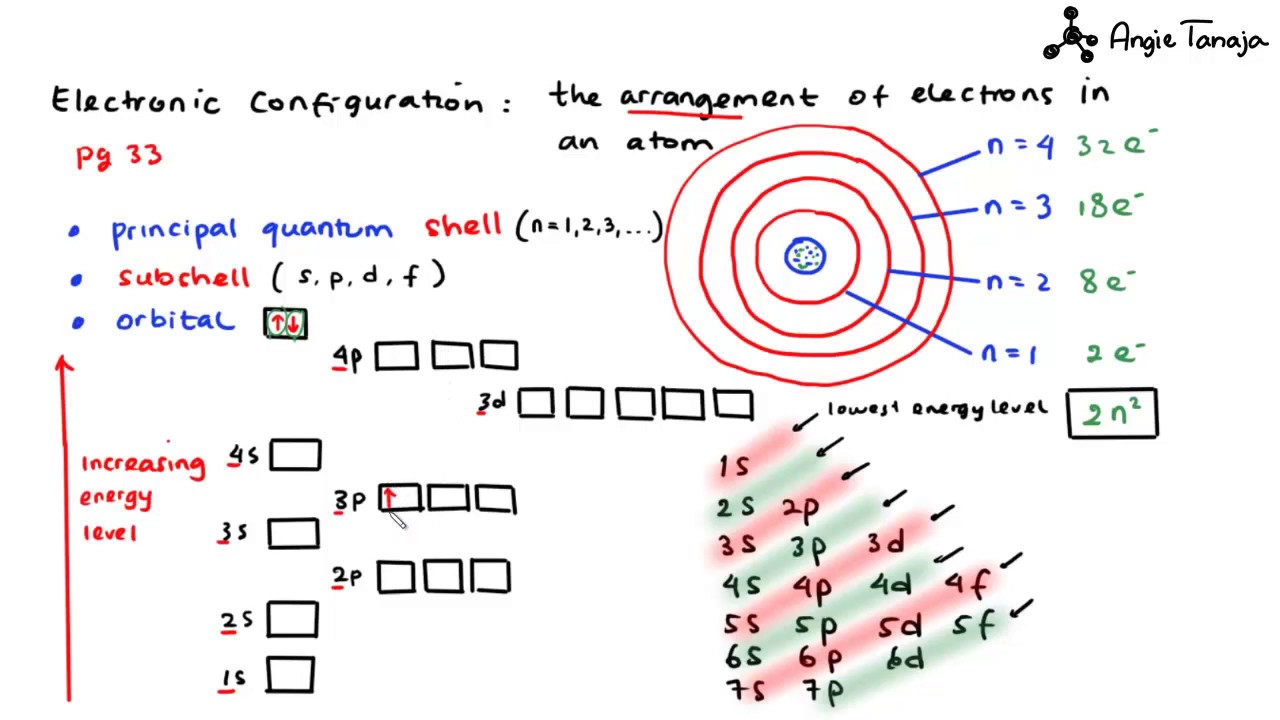
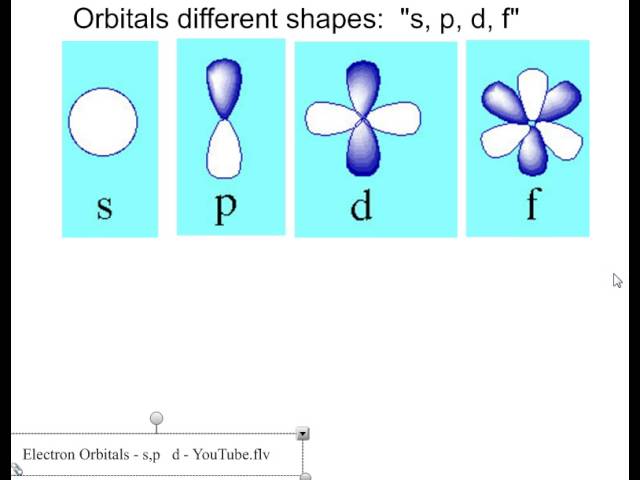
S d and p orbitals. At the third level there is a set of five d orbitals with complicated shapes and names as well as the 3s and 3p orbitals 3p x 3p y 3p z. In addition to s and p orbitals there are two other sets of orbitals which become available for electrons to inhabit at higher energy levels. The s orbitals are spherical while p orbitals are polar and oriented in particular directions x y and z. The simple names s orbital p orbital d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ 0 1 2 and 3 respectively.
It may be simpler to think of these two letters in terms of orbital shapes d and f aren t described as readily. An s orbital is spherical with the nucleus at its centre a p orbitals is dumbbell shaped and four of the five d orbitals are cloverleaf shaped. These names together with the value of n are used to describe the electron configurations of atoms. At the third level there is a set of five d orbitals with complicated shapes and names as well as the 3s and 3p orbitals 3px 3py 3pz.
All levels except the first have p orbitals. S s orbital overlap formation of h 2 molecule. The fifth d orbital is shaped like an elongated dumbbell with a doughnut around its middle. In the case of s and p orbitals there can be three types of overlap.